Vero Cell Vaccine Covid-19 Wikipedia, Covid 19 Vaccine Clinical Research Wikipedia
It was Phase III clinical trialled in Brazil Chile Indonesia the Philippines and Turkey and relies on traditional technology similar to BBIBP-CorV and Covaxin other inactivated-virus COVID-19 vaccines. Recommended schedule 2 doses 05 mL each at a recommended interval of 2 to 4 weeks.
How Covid 19 Vaccines Can Shape China And India S Global Influence
Sinopharm die zulassung für seinen impfstoff mit dem.
Vero cell vaccine covid-19 wikipedia. The COVID-19 Vaccine Vero Cell Inactivated is made from the SARS-CoV-2 19nCoV-CDC-Tan-HB02 strain which is inoculated on the Vero cells for culturing harvesting β-propiolactone-inactivation concentration and purification then followed. Vaccinating outbound workers with China-made Vero Cell shots adds to complication officials say. WHO Package Insert vaccine vials WHO Package Insert pre-filled syringes.
The vaccine which is developed by Chinas Sinopharm is not recognised by most labour destination countries. The Sinopharm COVID-19 vaccine vero cell inactivated vaccine resource includes key information on the vaccine specific requirements. One of the earliest papers to identify the pathogen behind the 2003 SARS outbreak for instance used this kind of cell.
The company is in the process of setting up a second plant at its Genome Valley facility in Hyderabad to make Covaxin. Recommendation for an Emergency Use Listing EUL of SINOVAC Covid-19 vaccine Vero cell Inactivated - CoronaVac TM Published. Vero cell vaccine covid-19.
An oral vaccination against coronavirus. It was approved for use in Russia in February 2021 being the third COVID-19 vaccine to get approval in Russia. The vaccine candidate is produced with Bharat Biotechs in-house vero cell manufacturing platform that has the capacity to deliver about 300 million doses.
Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject. COVID-19 Vaccine Vero Cell Inactivated CoronaVac COVD1 Vaccine Explainer 11 JUNE 1 Recommended for age 18 to 59 years of age as per WHO EUL Based on the data reviewed WHO SAGE recommends use in persons aged 18 years and above. VLA2001 COVID-19 Vaccine Description.
The vaccine called Vero. At the start date. Vero cell COVID-19 VACCINE being administered across Nepal Thousands of people all 64 years old lined up at vaccination centers even before they opened.
Vero cell vaccine sinopharm or sinovac. When introduced into human tissue the RNA contained in the vaccine acts as messenger RNA mRNA to cause cells to build the SARS-CoV-2. Nepal has granted emergency approval to the chinese vero cell vaccine given the active caseload that stands at 277944 on monday while 273240 people have recovered in nepal.
Valneva SEs VLA2001 VLA2101 is a vero-cell-based highly purified inactivated vaccine candidate against the novel beta coronavirus SARS-CoV-2 that causes COVID-19 in humans. VLA2001 consists of inactivated whole virus particles of SARS-CoV-2 with high S-protein density combined with two adjuvants alum and CpG 1018. Several COVID-19 vaccines including the PfizerBioNTech and Moderna vaccines have been developed to use RNA to stimulate an immune response.
КовиВак is an inactivated virus-based COVID-19 vaccine developed by the Chumakov Centre which is an institute of the Russian Academy of Sciences. It obtained a permission for phase III clinical trial on 2 June 2021. CoronaVac also known as the Sinovac COVID-19 vaccine is an inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech.
This means that a harmless transport virus brings non-replicable surface proteins of the SARS-CoV-2 pathogen to the cells. COVID-19 voluit coronavirus disease 2019 is een infectieziekte die wordt veroorzaakt door het SARS-CoV-2-virus behorende tot de coronavirussenDe voorheen onbekende ziekte werd eind 2019 voor het eerst opgemerkt in China en verspreidde zich vervolgens in drie maanden tijd naar andere delen van de wereldIn 2020 leidde dit tot de coronapandemie. The woes of tens of thousands of Nepali job seekers whose travel and job plans have been relying on Covid-19 vaccination are not over.
Vero cells have at times been indispensable to the study of coronaviruses.
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters

Covid 19 Vaccine Clinical Research Wikipedia

Sinopharm Bibp Covid 19 Vaccine Wikipedia

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
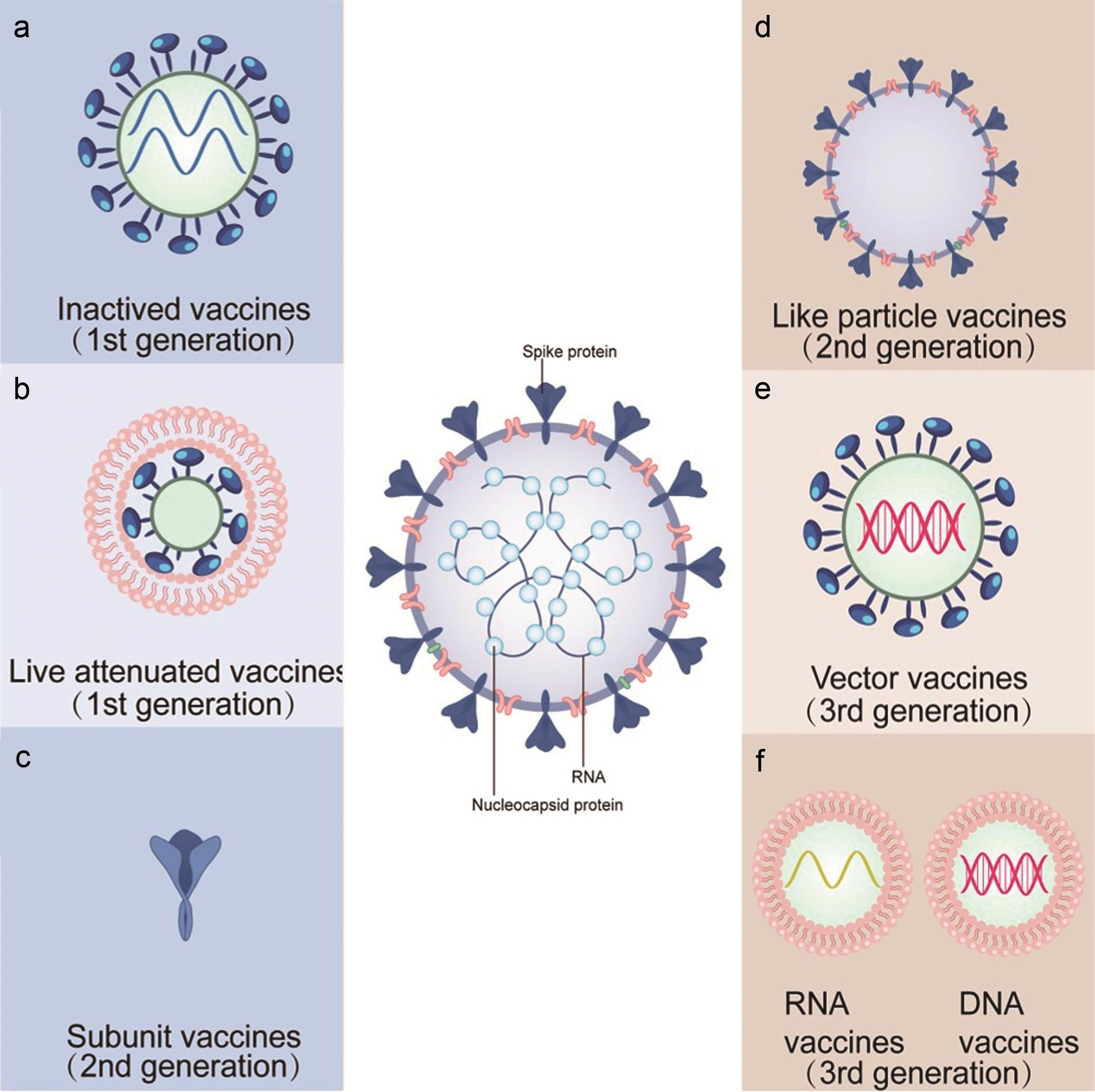
Research Development And Application Of Covid 19 Vaccines Progress Challenges And Prospects
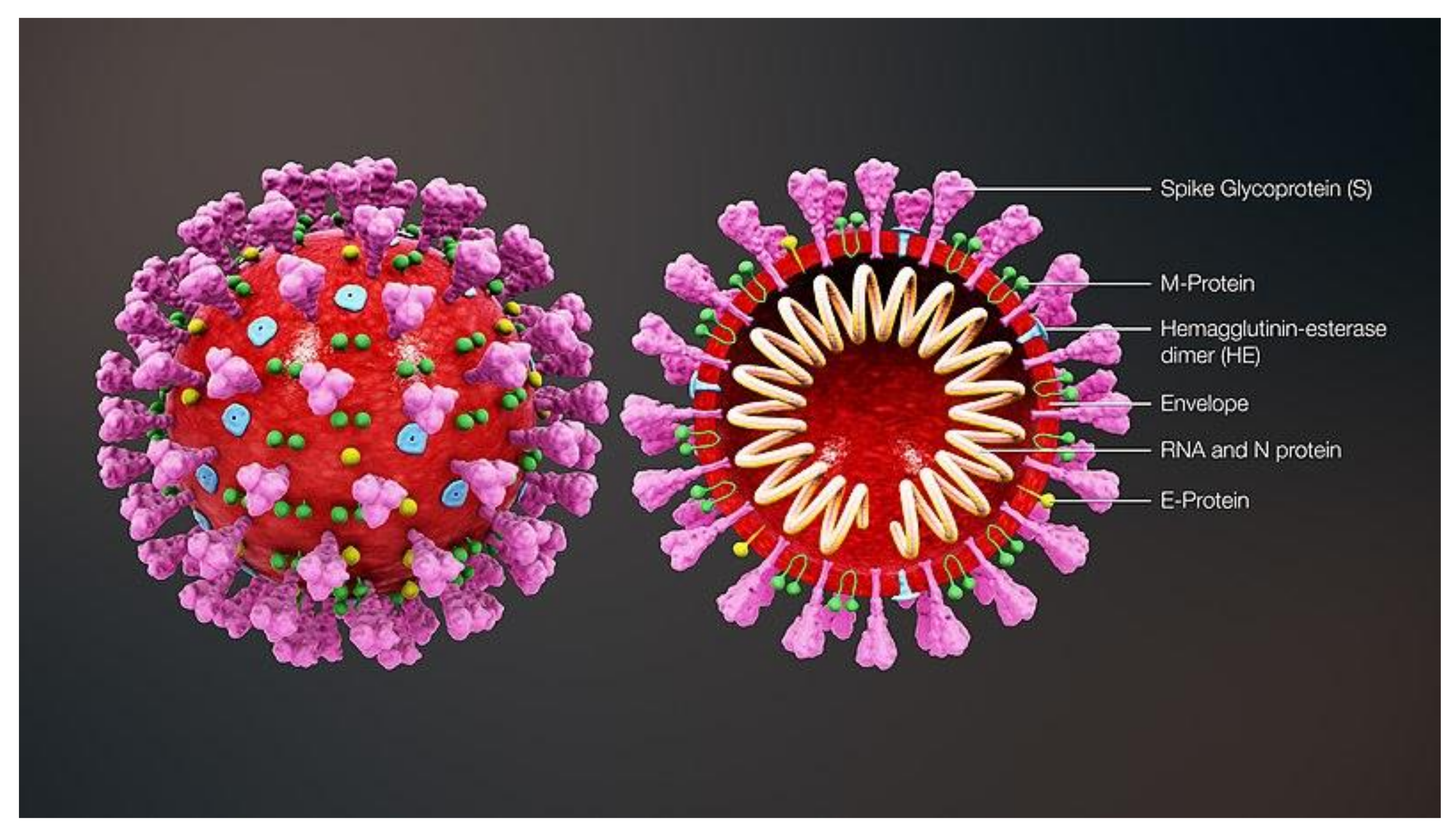
Jcm Free Full Text Vaccines And Therapies In Development For Sars Cov 2 Infections Html
/cloudfront-us-east-2.images.arcpublishing.com/reuters/K7IMX7V5NZOOVGSOPKVPNUA6MY.jpg)
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters

Covaxin India S First Indigenous Covid 19 Vaccine Bharat Biotech

Sars Cov 2 Impfstoff Wikipedia

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

Covaxin India S First Indigenous Covid 19 Vaccine Bharat Biotech
Austria Observatory On Border Crossings Status Due To Covid 19 Unece Wiki
Preliminary Results Show Chinese Covid 19 Vaccine Safe The Lancet Cgtn



