Vero Cell Vaccine Duration Between Two Doses | Phase I Ii Study Of Covid 19 Rna Vaccine Bnt162b1 In Adults Nature
Vaccine Placebo NMPA definition 854953110 1684870223 507 359 620 Protocol definition 804953104 1704870227 541 401 648 As of 16 th Dec 2020 the average follow-up time after the 2 nd dose was 703 256 days and the median follow-up duration was 730 days. In light of the observation that two-dose efficacy and immunogenicity increase with a longer inter-dose interval WHO recommends an interval of 8 to 12 weeks between the doses.

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Serum neutralizing antibodies were measured pre-vaccination and 28 days after each dose.

Vero cell vaccine duration between two doses. Number and percentage of local and systemic adverse events occurring within 7 days after a booster dose of inactivated Vero cell culture--derived Japanese encephalitis vaccine JE-VC manufactured as Ixiaro administered 15 months after dose 1 of a 2-dose primary JE-VC series. The World Health Organization WHO recommends an interval of 3 to 4 weeks between doses. A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy.
If anything the vaccine choice has added to the complication. At the start of the second wave of the Covid-19 epidemic the Joint Committee on Vaccination and Immunisation JCVI decided to recommend 12 weeks between two doses for the two vaccines that were. In this clinical trial low dosage 300SUdose and medium dosage 600SUdose with two-dose immunization scheduled at 28-day intervals will be adapted.
Inactivated SARS-CoV-2 vaccine Vero cell. If the second dose is inadvertently administered less than four weeks after the first the dose does not need to be repeated said the WHOs strategic advisory group of experts on immunisation. There is no such luxury with the Vero Cell shots.
COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date. China donated Covid-19 vaccine Vero Cell arrives in Kathmandu. Research has shown that a 12-week interval between AstraZeneca vaccine doses offers optimal protection The UK has cut the wait between doses down to eight weeks in.
The Geometric Mean Titer GMT of anti-SARS-CoV-2 neutralizing antibody Time Frame. Recommended schedule 2 doses 05 mL each at a recommended interval of 3 to 4 weeks. The vaccine is given by intramuscular injection into the deltoid muscle.
However further studies are needed before this can happen. In China the vaccine has received emergency approval. Epektibo ang ating mga bakuna at napakahalaga na dalawang doses ang maibigay sa atin para maabot natin yung magandang efficacy rate Our vaccines are effective and it is very important that we complete the two doses so that we can have a good efficacy rate said DOH Epidemiology Bureau director Dr.
26 COVID-19 Vaccine Vero Cell Inactivated Sinopharm CO1 accne Explaner 4 MAY 1 Recommended for age 18 years of age and above Vaccination of children or adolescents below the age of 18 years is not routinely recommended although the studies are underway. Alethea De Guzman during the Malacañang press briefing on Thursday July 15. Sinovac hopes to increase efficacy by prolonging the interval between doses.
Indian govt revises gap-duration between two doses of Covid vaccine. A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy of 79 against symptomatic SARS-CoV-2. 612 95 CI 410757 18 years against COVID-19 with two standard doses 900 95 CI 674-970 against COVID-19 with a low dose followed by a standard dose 704 958 548-806 overall vaccine efficacy against COVID-19 across both dosing groups 4 23 April 20204 November 2020.
The initial course consists of two doses and there is no evidence that a third booster dose dose is needed. Sinopharm Vero Cell Inactivated Covid 19 Vaccine. For individuals who have been potentially exposed to the virus four doses over two weeks are recommended as well as an.
28 days 3rd month 6th month 9th month and 12th month after 2 doses of immunization The 4-fold increase rate of anti-SARS-CoV-2 neutralizing antibody Time Frame. The ministrys clarification came in. 4μgdose for per human use 05 mL dose.
NoTotal subjects Local adverse events. Non-inferiority was evaluated for seroprotection rate and geometric mean titer GMT between previously vaccinated participants post-dose 1 and vaccine-naïve participants post-dose 2. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
This is another complication if Vero Cell shots are given to migrant workers said Shrestha. 4000 participants per arm. 28 days 3rd month 6th month 9th month and 12th month after 2 doses of immunization.
Three doses of the vaccine are given over a one-month period on days zero seven and either twenty-one or twenty-eight. With the Johnson Johnson vaccine Shrestha said workers could take the one-time jab rest in Nepal for 14 days and fly to labour destination countries.

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
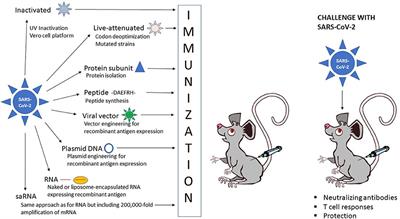
Frontiers The Current Status Of Covid 19 Vaccines Genome Editing
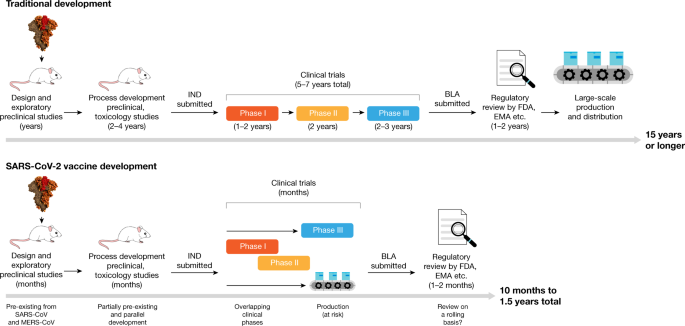
Sars Cov 2 Vaccines In Development Nature

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
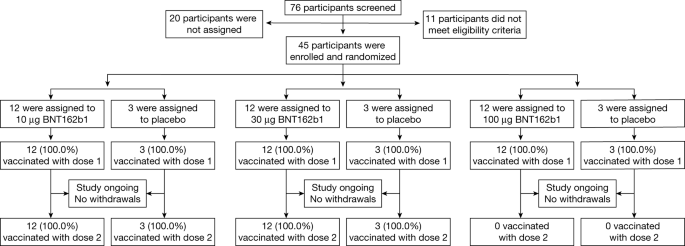
Phase I Ii Study Of Covid 19 Rna Vaccine Bnt162b1 In Adults Nature
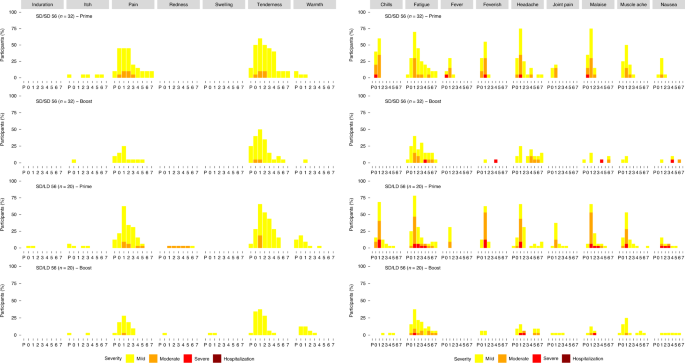
Phase 1 2 Trial Of Sars Cov 2 Vaccine Chadox1 Ncov 19 With A Booster Dose Induces Multifunctional Antibody Responses Nature Medicine
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Https Www Who Int Docs Default Source Coronaviruse Act Accelerator Covax 21221 Sinovac Vaccine Explainer Pdf Sfvrsn 69283a08 3 Download True
Vaccines Free Full Text Development And Evaluation Of Vero Cell Derived Master Donor Viruses For Influenza Pandemic Preparedness Html

Covid 19 Chinese Official Says Homegrown Vaccines Not Very Powerful Euronews

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld

Coronavirus How Effective Are The Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 01 02 2021
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 4 Sage29apr2021 Sinovac Pdf
Explainer Are Chinese Covid 19 Shots Effective Against The Delta Variant Reuters
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu