Vero Cell Vaccine Effective Rate : Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet
A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy of 79 against symptomatic SARS-CoV-2 infection 14 or more days after the second dose. The use of cell-culture technologies for the manufacture of influenza vaccines might contribute to improved strain selection and robust vaccine supplies.
Vaccines Free Full Text Development And Evaluation Of Vero Cell Derived Master Donor Viruses For Influenza Pandemic Preparedness Html
A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy of 79 against symptomatic SARS-CoV-2 infection 14 or more days after the second dose.
Vero cell vaccine effective rate. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru - Full Text View. So far Vero is the only Chinese vaccine for which the manufacturer has published official data. The findings were reported in the May 26 Journal of the American Medical Association.
Purified Vero cell rabies vaccine is safe carries a very low adverse reaction rate and is effective in preventing rabies in severely exposed subjects when used with human or. Sinovac had a 51 efficacy against symptomatic cases of COVID-19 according to the WHO. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above - Full Text View.
Based on a review of data. Vaccine efficacy against hospitalization was 79. A leading US medical journal found that the two vaccines prevented symptomatic infections by 728 per cent and 781 per cent largely in line with what the state-owned drug maker previously announced.
The sample size of some patients is small which needs further study. On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation. While AstraZeneca claimed that the vaccine was 70-percent effective it was later disclosed that the effectiveness was 62 percent in people who received two full doses and closer to 90 percent in.
Here is what you need to know. EMAs human medicines committee has started a rolling review of COVID-19 Vaccine Vero Cell Inactivated developed by Sinovac Life Sciences Co LtdThe EU applicant for this medicine is LifeOn Srl. In April 2021 a study by the Abu Dhabi Public Health Centre found the vaccine was 93 effective in preventing hospitalization and 95 effective against admission to intensive care.
Comparative study of the safety and protective value in pre-exposure use of rabies vaccine cultivated on human diploid cells HDCV and of the new vaccine grown on vero cells. Final numerical rating of quality of evidence 4 s Statement on quality of evidence Evidence supports a high degree of confidence that the true effect lies close to that of the estimate of effect on health outcome Conclusion Inactivated Vero cell-derived JE vaccines elicit seroprotective neutralizing antibody titres. BHK-21A and 293 cells grown as single-cell suspension in serum free medium were both suited to produce PPR vaccine with productivities similar to Vero cells namely 106TCID50mL.
COVID-19 Vaccine Vero cell. The interim recommendations and the. The study found no deaths related to COVID-19 in patients who received both doses.
We investigated the safety immunogenicity and protective efficacy of a Vero-cell-culture-derived influenza vaccine and assessed the correlation between vaccine efficacy and haemagglutination inhibition antibody titre. How efficacious is the vaccine. Rate 100 person year CoronaVac 0 6550 000 28369 000 Placebo 6 3445 017 16651 363 Total 6 9995.
Precondition had no significant effect on the Efficacy. The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine Sinovac-CoronaVac developed by SinovacChina National Pharmaceutical Group. Vaccine efficacy against hospitalization was 79.
Bahri F Letaief A Ernez M Elouni J Chekir T Ben Ammou S Jemni L 1996. The CHMPs decision to start the rolling review is based on preliminary results from laboratory studies non-clinical data and clinical studies. Vaccinating outbound workers with China-made Vero Cell shots adds to complication officials say.
It was unknown how many people were included in the research. The vaccine which is developed by Chinas Sinopharm is not recognised by most labour destination countries. This article provides a summary of the interim recommendations.
The woes of tens of thousands of Nepali job seekers whose travel and job plans have been relying on Covid-19 vaccination are not over. The vaccine also had a 100 efficacy against severe cases and hospitalization in the studied populations per. Ajjan N Pilet C 1989.
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
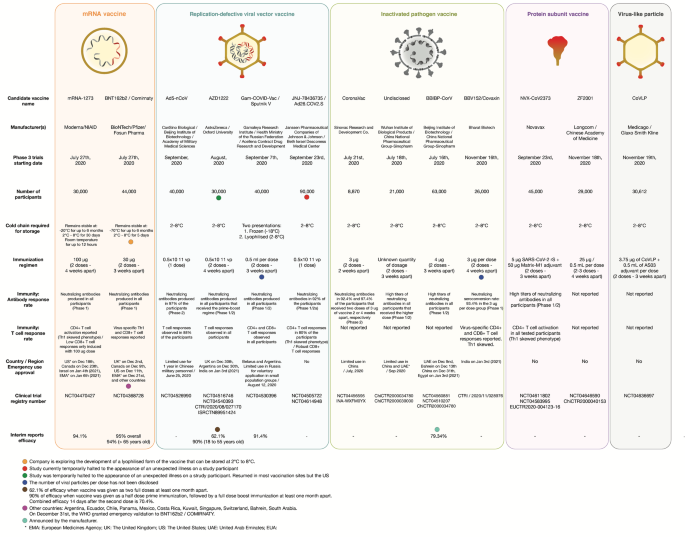
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy
Https Www Who Int Immunization Policy Position Papers Je Grad Inactivated Effectiveness Pdf

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Expert Chinese Vaccines Effective Against Covid 19 Delta Variant Cgtn

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News

Chinese Made Covid 19 Vaccine Nearly 90 Effective Uae Says Voice Of America English
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet

Vaccine Diplomacy China And Sinopharm In Africa Council On Foreign Relations

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet
Study Sinopharm Vaccine Over 90 Effective At Preventing Hospitalization Dhaka Tribune
Doubts Over China Vaccines Effectiveness Mar Production Push Nikkei Asia
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn

